Tag: FDA
-
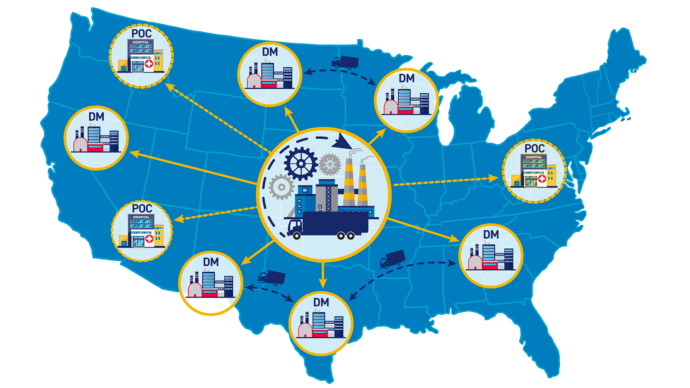
Discussion Paper: Distributed Manufacturing and Point-of-Care Manufacturing of Drugs; Request for Information and Comments FDA | October 14, 2022 This discussion paper presented areas associated with DM and POC manufacturing that FDA has identified for consideration as FDA evaluates existing risk-based regulatory framework as it applies to these technologies. CDER scientific and policy experts identified…